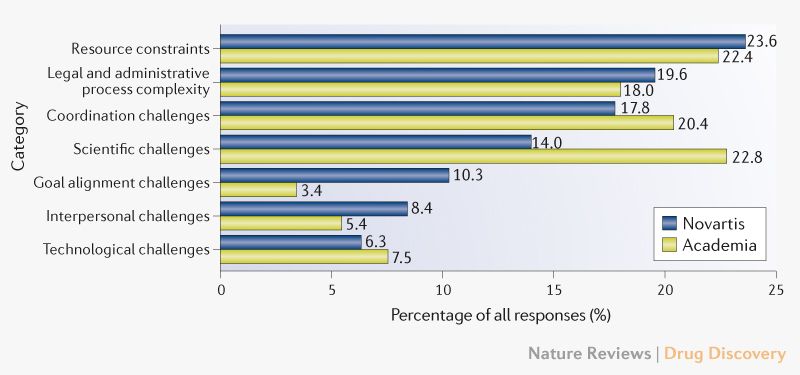
Research collaboration between pharmaceutical companies and academic institutions are replete with challenges. These challenges are:
- Resource constraints, the most frequently mentioned category, refers to the limited availability of human, monetary and organizational resources
- Legal and administrative process complexity includes challenges due to ‘paperwork’, internal approval processes, ethical reviews, and contract negotiations
- Coordination challenges relate to difficulties in teamwork, the frequency and quality of communication, the coordination of tasks and the exchange of goods and knowledge
- Scientific challenges arise from negative results, issues with the scientific methodology and difficulties in interpreting data
- Goal alignment challenges, relates to diverging expectations and goals among project members and insufficient priority of the project in the partnering organizations
- Interpersonal challenges include issues related to differences in project members’ individual attitudes, behaviours and interests, a lack of trust in the partner, a lack of commitment by single project members and interpersonal conflict
- Technological challenges arise from scarce knowledge on new technologies and methods, uncertainty in the technical feasibility of methods and unreliable experimental techniques.