Since beginning full operations in late 2011, Vetter’s US early-stage development site has been expanding to help meet growing customer demands. A significant level of expansion activities are nearing completion at its US clinical manufacturing facility located at the Illinois Science & Technology Park. The ongoing growth of the facility will help satisfy existing and ever-increasing future customer requirements as well enable meet the complex needs of newer drug molecules like peptides or antibodies, many which need refrigeration or freezing. New offices with 45 work stations, conference rooms and an archive room are also included. To support the increase in customer projects, a permanent second work shift will be added in Visual Inspection over the next months. A second shift in Quality Oversight is also planned.
“This variety of activities is a further proof point of Vetter’s consistent strategic approach to stay ahead of the market by focusing on the important service needs of our customers during their drug development journey; promptness; flexibility; high yield of their valuable API and, of course high quality,” explains Dr. Claus Feussner, Senior Vice President of Vetter Development Service. For more details, click here to access the entire press release.
With the new additions, most of which are expected to be completed by April, the site will increase its storage space by an additional 3,100 sq. ft. The new storage includes a 2,500 sq. ft. freezer farm as well as a planned 600 sq. ft. walk-in refrigerator. In total, 6,800 sq. ft. of storage space will result. A second extension, now in the final planning stage, will include an additional 1,500 sq. ft. of room temperature and freezer space. “When completed, the facility will have more than double the overall storage space we have currently available. This extensive expansion of freezer and refrigeration storage space represents the ongoing evolution in our Chicago business,” summarizes Dr. Susanne Lemaine, Vice President Vetter Development Service Chicago.


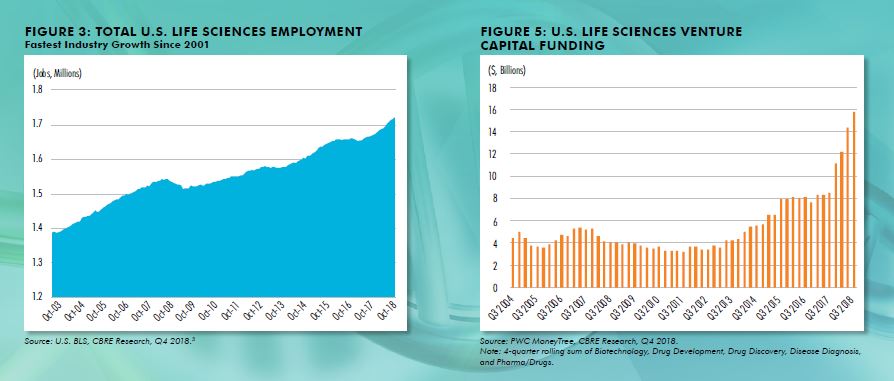 a strong acceleration from the slow,but temporary, growth in early 2017 and well-above the 1.7% increase in total U.S. nonfarm employment over the same period. Employment in the industry has grown 42% over the past 20 years. All sectors of life sciences (Manufacturing, Testing Labs, R&D) are growing, but R&D continues to outperform. The key subsector that is driving growth, even within the larger R&D sector, is research & development in biotechnology. This subsector grew at an annual pace of 6.2% in Q3 2018, far greater than other sectors of life sciences. Biotech R&D has grown 88% over the past 20 years.
a strong acceleration from the slow,but temporary, growth in early 2017 and well-above the 1.7% increase in total U.S. nonfarm employment over the same period. Employment in the industry has grown 42% over the past 20 years. All sectors of life sciences (Manufacturing, Testing Labs, R&D) are growing, but R&D continues to outperform. The key subsector that is driving growth, even within the larger R&D sector, is research & development in biotechnology. This subsector grew at an annual pace of 6.2% in Q3 2018, far greater than other sectors of life sciences. Biotech R&D has grown 88% over the past 20 years.
 Join Charles River on April 2-3. Genetic toxicology testing is required for all classes of chemicals and drugs, but its conduct can differ from compound to compound. A poor strategy can result in repeated studies, failure to meet timelines, or worse – abandonment of a once promising program.
Join Charles River on April 2-3. Genetic toxicology testing is required for all classes of chemicals and drugs, but its conduct can differ from compound to compound. A poor strategy can result in repeated studies, failure to meet timelines, or worse – abandonment of a once promising program.